
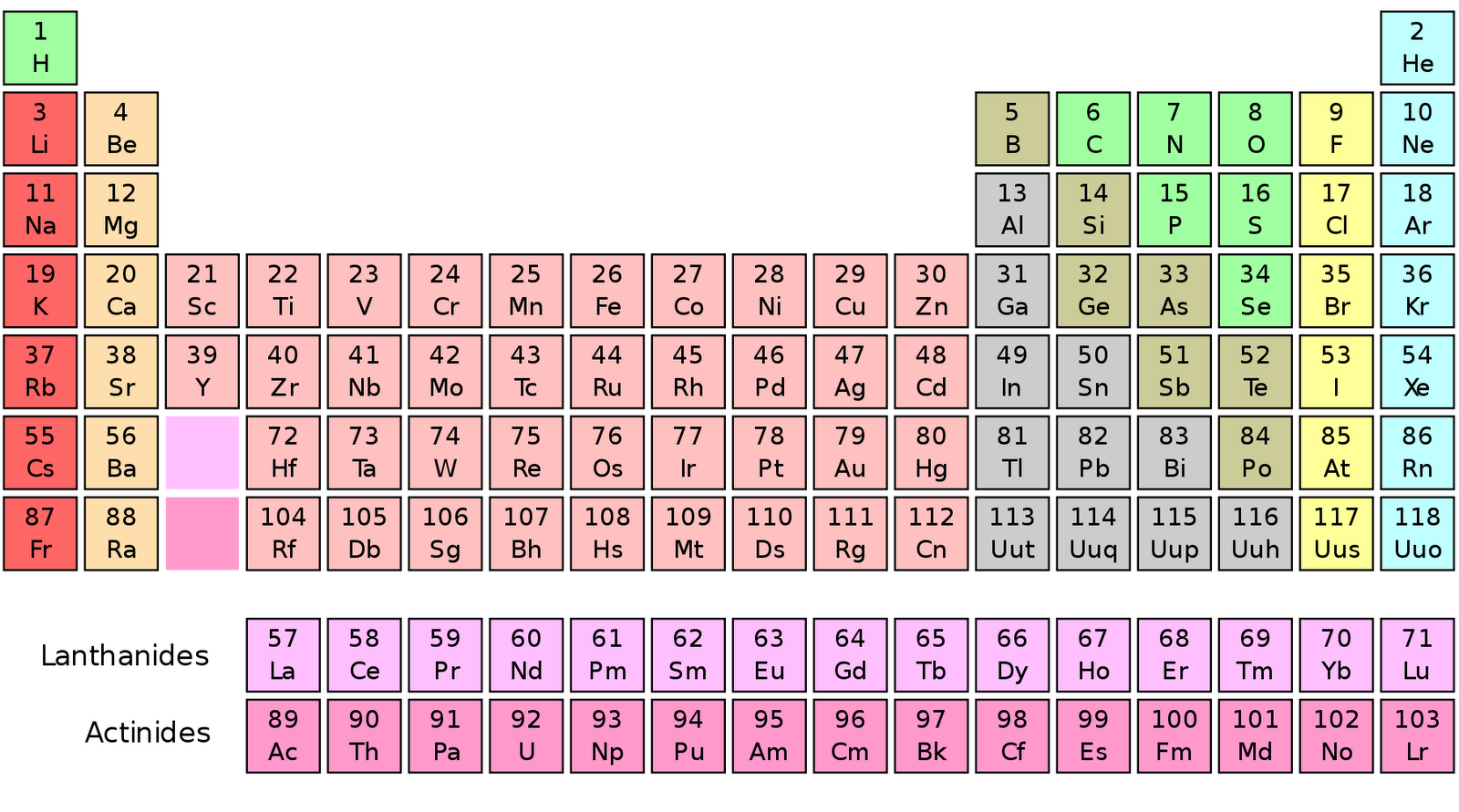
Columns ( groups) are determined by the electron configuration of the atom elements with the same number of electrons in a particular subshell fall into the same columns (e.g. A new row ( period) is started when a new electron shell has its first electron. In the standard periodic table, the elements are listed in order of increasing atomic number Z. When atomic mass is shown, it is usually the weighted average of naturally occurring isotopes but if there are none, the mass of the most stable isotope usually appears, often in parentheses. Isotopes are never separated in the periodic table they are always grouped together under a single element. For example, carbon has three naturally occurring isotopes: all of its atoms have six protons and most have six neutrons as well, but about one per cent have seven neutrons, and a very small fraction have eight neutrons. Most elements have multiple isotopes, variants with the same number of protons but different numbers of neutrons. Many alternative representations of the periodic law exist, and there is some discussion as to whether there is an optimal form of the periodic table.Įach chemical element has a unique atomic number ( Z) representing the number of protons in its nucleus. Some scientific discussion also continues regarding whether some elements are correctly positioned in today's table. It is not yet known how far the table will go beyond these seven rows and whether the patterns of the known part of the table will continue into this unknown region. Today, while all the first 118 elements are known, thereby completing the first seven rows of the table, chemical characterisation is still needed for the heaviest elements to confirm that their properties match their positions. In nature, only elements up to atomic number 94 exist to go further, it was necessary to synthesise new elements in the laboratory. The periodic table continues to evolve with the progress of science. The periodic table and law are now a central and indispensable part of modern chemistry. Seaborg's discovery that the actinides were in fact f-block rather than d-block elements. A recognisably modern form of the table was reached in 1945 with Glenn T. It was explained early in the 20th century, with the discovery of atomic numbers and associated pioneering work in quantum mechanics both ideas serving to illuminate the internal structure of the atom. The periodic law was recognized as a fundamental discovery in the late 19th century. As not all elements were then known, there were gaps in his periodic table, and Mendeleev successfully used the periodic law to predict some properties of some of the missing elements. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869 he formulated the periodic law as a dependence of chemical properties on atomic mass. Nonmetallic character increases going from the bottom left of the periodic table to the top right. Metallic character increases going down a group and decreases from left to right across a period. Vertical, horizontal and diagonal trends characterize the periodic table.
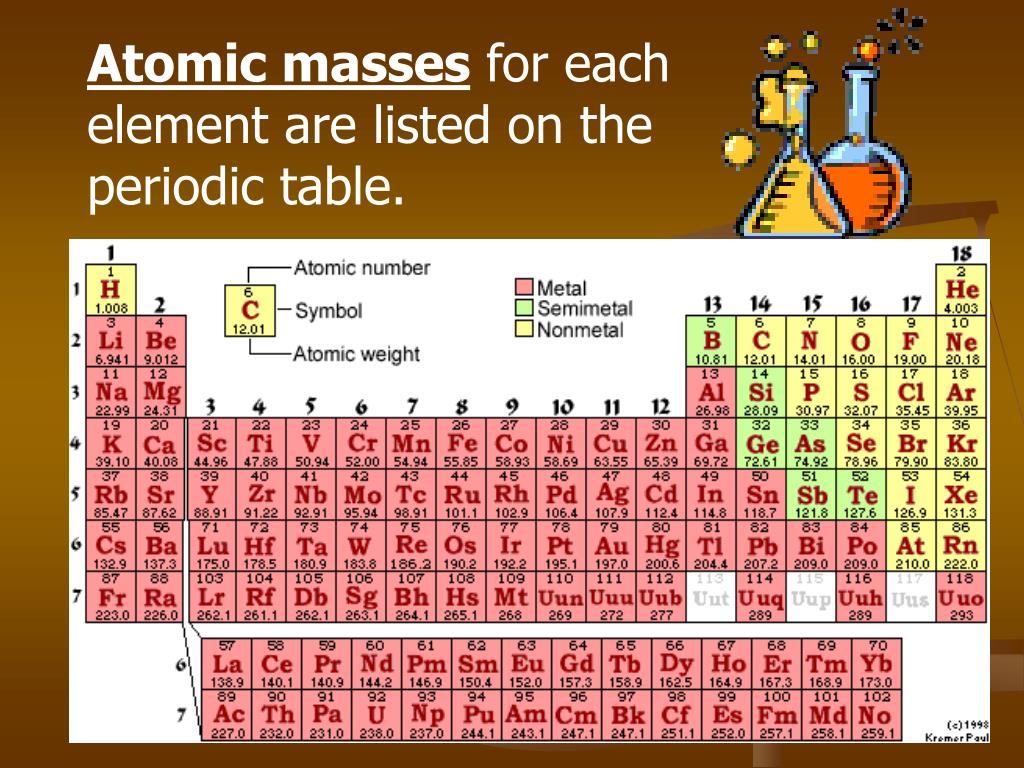
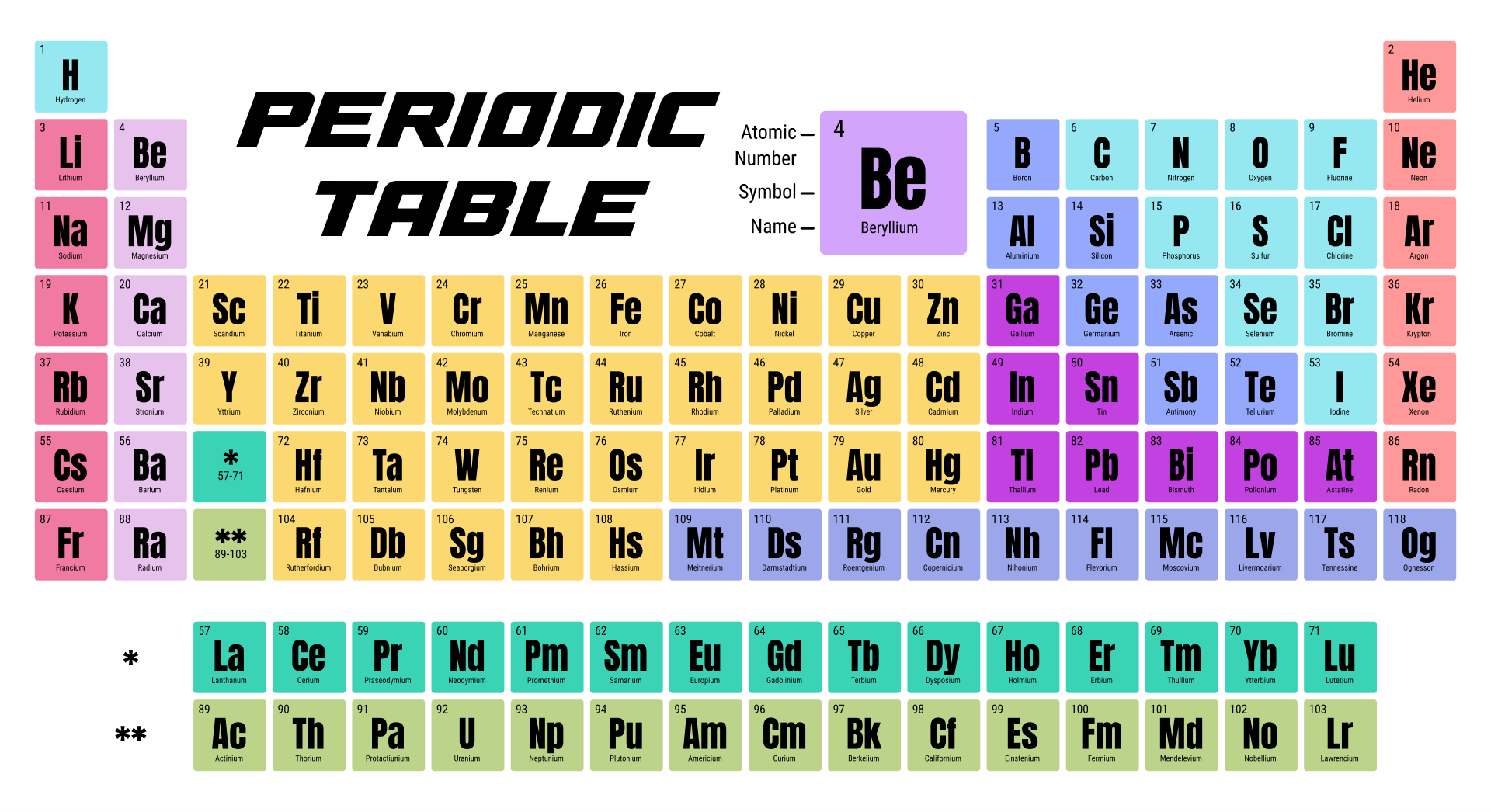
Elements in the same group tend to show similar chemical characteristics. The table is divided into four roughly rectangular areas called blocks. It is a depiction of the periodic law, which says that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. It is an organizing icon of chemistry and is widely used in physics and other sciences. The periodic table, also known as the periodic table of the elements, arranges the chemical elements into rows (" periods") and columns (" groups").


 0 kommentar(er)
0 kommentar(er)
